CAR001 新一代的免疫治療製劑
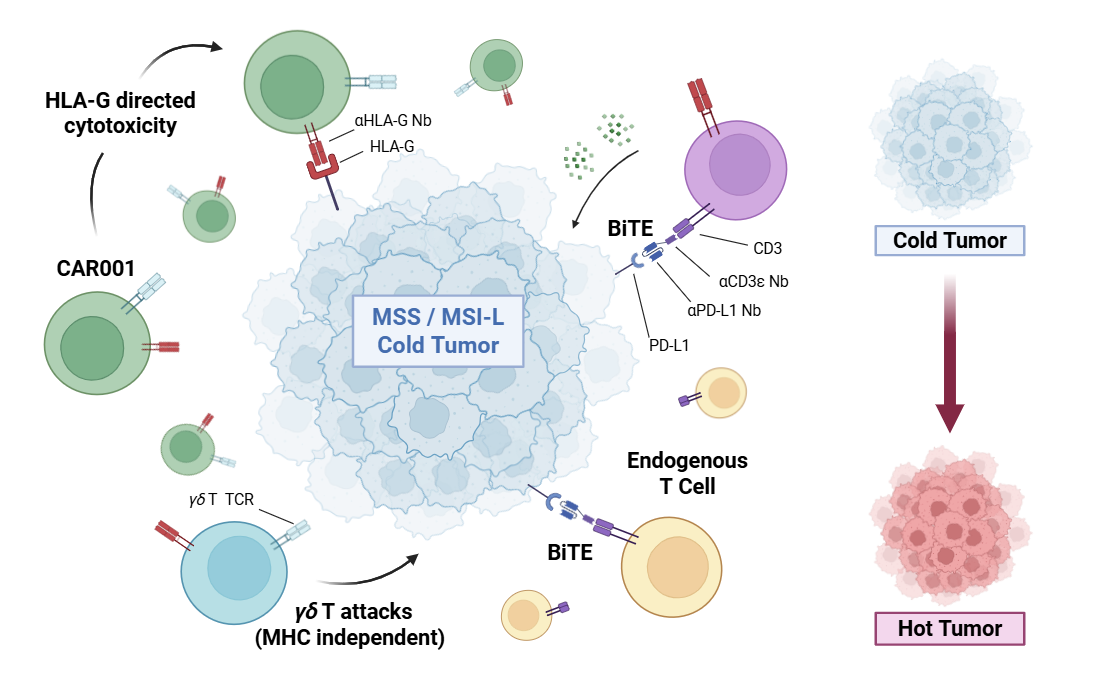
CAR001 是一種 mRNA 工程的 γδ T 細胞載體,融合了嵌合抗原受體(CAR)與 γδ T 細胞本身的先天免疫特性,可透過靜脈注射給藥,賦予患者強大的抗腫瘤活性與組織浸潤能力。
CAR001 is an allogeneic, off-the-shelf γδ T cell therapy engineered via mRNA technology to transiently express a chimeric antigen receptor (CAR). Administered intravenously, CAR001 is intended to deliver potent anti-tumor activity with improved safety and manufacturability, particularly for patients with relapsed or refractory solid tumors.
CAR001 是一種 mRNA 工程的 γδ T 細胞載體,融合了嵌合抗原受體(CAR)與 γδ T 細胞本身的先天免疫特性,可透過靜脈注射給藥,賦予患者強大的抗腫瘤活性與組織浸潤能力。
CAR001 is an allogeneic, off-the-shelf γδ T cell therapy engineered via mRNA technology to transiently express a chimeric antigen receptor (CAR). Administered intravenously, CAR001 is intended to deliver potent anti-tumor activity with improved safety and manufacturability, particularly for patients with relapsed or refractory solid tumors.

人類白血球抗原 G(Human Leukocyte Antigen G,HLA-G)為一種在多種實體腫瘤中新生表現的腫瘤相關抗原。
Human leukocyte antigen G (HLA-G) is a neo-expressed tumor-associated antigen (TAA) in a large proportion of solid tumors.
γδ T 細胞可直接誘導腫瘤細胞毒殺作用,並具備非主要組織相容性複合體的抗腫瘤活性,因而可降低移植物對宿主疾病(GvHD)的發生風險。
γδ T cells can induce cytotoxicity directly and also exhibit major histocompatibility complex (MHC)-independent anti-tumor activities, which reduces graft-versus-host disease (GvHD).
Bispecific T-cell Engager (BiTE)
BiTE 為一種人工設計的雙特異性抗體結構,可同時結合腫瘤細胞表面的 PD-L1 與 T 細胞上的 CD3ε,藉此引導 T 細胞精準攻擊腫瘤細胞
BiTE is an artificial bispecific antibody construct that simultaneously binds PD-L1 on tumor cells and CD3ε on T cells.
為何選擇 γδT 細胞 Why γδT Cells?
此外,γδ T 細胞具有先天與適應性雙重免疫能力,能同時利用天然細胞毒性與 CAR 介導的抗腫瘤機制,提高療效可能性。
CAR001 的設計與機制 CAR001 Design and Mechanism of Action
【CAR 與 mRNA 設計】
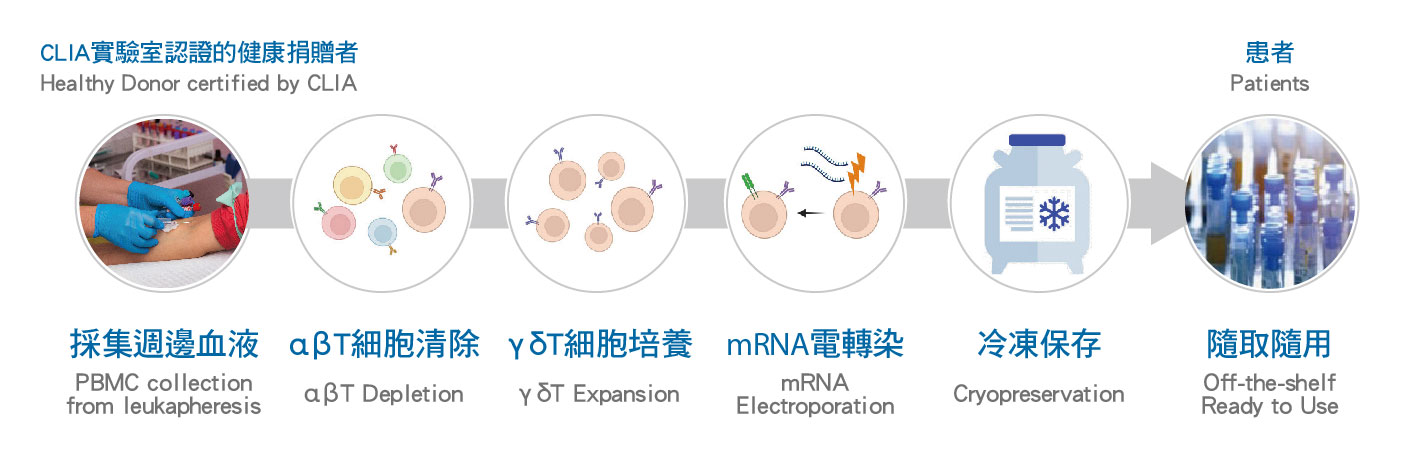
經CLIA實驗室認證的健康捐贈者的血液中採集γδ T細胞,然後進行mRNA轉染以引導CAR的表現。
經CLIA實驗室認證的健康捐贈者的血液中採集γδ T細胞,然後進行mRNA轉染以引導CAR的表現。
這種基於mRNA的工程化過程能夠精確調控CAR的表達量和持續時間,從而在抗腫瘤效力和安全性之間取得良好的平衡。
轉染後,我們的mRNA-γδ T細胞(CAR001)將進行冷凍保存,可作為隨去隨用的免疫細胞療法。
【mRNA-Based CAR Engineering】
Donor-derived γδ T cells are first collected from a healthy donor that are certified by CLIA lab, followed by mRNA transduction to introduce transient CAR expression.
This mRNA-based engineering process allows CAR expression to be precisely regulated in both level and duration, supporting a favorable balance between anti-tumor potency and safety.
After transduction, the engineered mRNA- γδ T cells (CAR001) are cryopreserved and available as an off-the-shelf cell therapy.