一、來源與取得方式 Source and Procurement
來源 Source:
新生兒出生後的臍帶
Umbilical cord collected after newborn delivery
取得流程 Procurement Process:
■ 取得捐贈臍帶(經捐贈者同意)
Collection of donated umbilical cord (with donor informed consent)
■ 無菌分離 Wharton’s Jelly、細胞分離、培養與擴增
Aseptic isolation of Wharton’s JellyCell isolation, culture, and expansion.
■ 品質與安全性檢驗(身分、純度、活性、無菌、內毒素等)
Quality and safety testing (identity, purity, potency/activity, sterility, endotoxin, etc.)
二、主要生物特性 Key Biological Characteristics
高增殖能力 High Proliferative Capacity:
相較成人來源 MSC,更易大量培養
Compared with adult-derived MSCs, they are more readily expanded to large quantities.
低免疫原性 Low Immunogenicity:
■ 低表現 HLA-DR
Expression of HLA-DR
■ 適合異體(allogeneic)使用
Suitable for allogeneic use
多向分化潛能 Multilineage Differentiation Potential:
■ 可分化硬骨、軟骨、脂肪等間質細胞
Capable of differentiating into osteogenic, chondrogenic, adipogenic, and other mesenchymal lineages.
旁分泌作用強 Strong Paracrine Activity:
■ 可分泌多種生長因子與細胞激素
Secretes a wide range of growth factors and cytokines.
三、作用機轉 Mechanism of Action
臍帶間質幹細胞主要透過調節而非取代的方式發揮療效:
Umbilical cord–derived mesenchymal stem cells (UC-MSCs) exert their therapeutic effects primarily through immunomodulation rather than direct cell replacement:
免疫調節 Immunomodulation:
■ 抑制過度活化的 T 細胞、B 細胞
Suppression of overly activated T cells and B cells
■ 調節巨噬細胞與樹突細胞反應
Regulation of macrophage and dendritic cell responses
抗發炎作用 Anti-inflammatory Effects:
■ 降低發炎性細胞激素組織修復與保護
Reduction of pro-inflammatory cytokines
■ 分泌神經保護與血管新生相關因子
Secretion of neuroprotective and angiogenic factors
■ 改善微環境、促進自體修復
Improvement of the local microenvironment and promotion of endogenous tissue repair
四、臨床應用領域 Clinical Application Areas
臍帶間質幹細胞應用於多種未被滿足醫療需求疾病,包括:
Umbilical cord–derived mesenchymal stem cells (UC-MSCs) have been applied to a range of diseases with significant unmet medical needs, including:
心血管疾病 Diseases:
■ 急性心肌梗塞
Acute myocardial infarction (AMI)
神經系統疾病 Neurological Disorders:
■ 中風、多發性硬化症(MS)
Stroke, multiple sclerosis (MS)
其他 Other Indications:
■ COVID-19
五、總結 Summary
臍帶間質幹細胞具備:
Umbilical cord–derived mesenchymal stem cells (UC-MSCs) offer the following advantages:
✔ 安全、倫理接受度高
High safety profile and strong ethical acceptability
✔ 良好免疫調節與修復潛力
Robust immunomodulatory and regenerative potential
✔ 適合異體即用型(off-the-shelf)產品開發
Well suited for allogeneic, off-the-shelf product development
因此,已成為再生醫療與細胞治療領域中極具前景的關鍵細胞來源之一。
Accordingly, UC-MSCs have emerged as one of the most promising and critical cell sources in the fields of regenerative medicine and cell therapy.
臍帶間質幹細胞治療流程 Umbilical Cord–Derived Mesenchymal Stem Cell Treatment Process
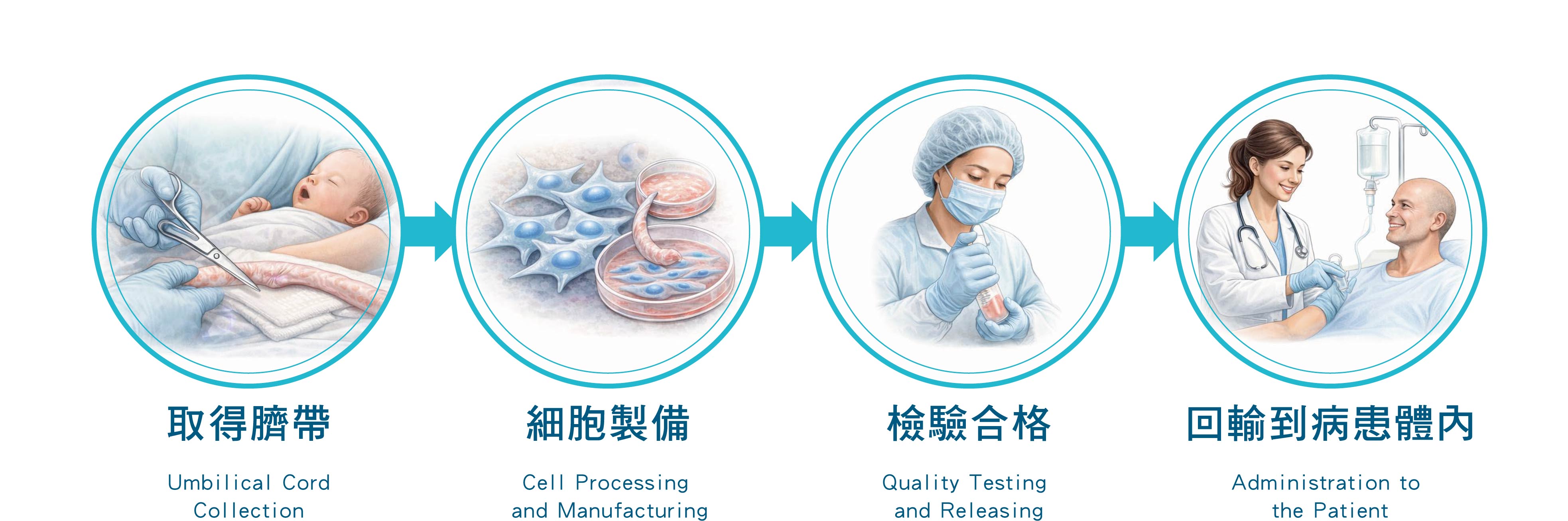
臍帶間質幹細胞治療流程說明
Overview of the Umbilical Cord–Derived Mesenchymal Stem Cell Treatment Process
本流程說明臍帶間質幹細胞自來源取得、製備、品質檢驗,至回輸病患體內之完整步驟,確保安全性與品質一致性。
This process outlines the complete workflow of umbilical cord–derived mesenchymal stem cells, from source collection and cell processing to quality testing and administration to patients, ensuring safety and consistency in product quality.
一、取得臍帶 Umbilical Cord Collection
於新生兒出生後,在取得產婦書面同意的前提下,由合格醫療人員無痛、無侵入性地採集臍帶組織。臍帶屬於醫療廢棄物來源,對母嬰安全無影響。
After neonatal delivery, umbilical cord tissue is collected painlessly and non-invasively by qualified medical personnel, following the obtainment of written informed consent from the mother. The umbilical cord is considered medical waste, and its collection poses no risk to either the mother or the newborn.
二、細胞製備 Cell Processing and Manufacturing
臍帶組織送至符合 GTP 規範之細胞製備實驗室,經組織處理、細胞分離與培養(使用無動物血清細胞培養),取得臍帶來源之間質幹細胞(UMSC01),並進行擴增與製劑化作業。
The umbilical cord tissue is transported to a GTP-compliant cell processing facility, where it undergoes tissue processing, cell isolation, and culture (using animal-component–free culture conditions) to obtain umbilical cord–derived mesenchymal stem cells (UMSC01). The cells are then expanded and formulated into the final cell product.
三、檢驗合格 Quality Testing and Releasing
細胞製品須通過嚴格品質檢驗,包括:
The cell product must pass stringent quality control testing, including:
■ 捐贈者經CLIA 認證實驗室檢驗合格
Donor screening with results approved by a CLIA-certified laboratory
■ 細胞身分鑑定(表面標記)
Cell identity confirmation (surface marker characterization)
■ 細胞活性與存活率
Cell potency/activity and viability assessment
■ 無菌、內毒素、黴漿菌檢測
Testing for sterility, endotoxin, and mycoplasma
■ 安全性與放行標準確認僅有檢驗合格之細胞製品方可使用於臨床。
Only cell products that meet all release specifications are eligible for clinical use.
四、回輸到病患體內 Administration to the Patient
經醫師評估後,將合格之臍帶間質幹細胞以輸注等方式回輸至病患體內,進行治療或臨床試驗應用。
Following physician evaluation, qualified umbilical cord–derived mesenchymal stem cells are administered to the patient, typically via infusion, for therapeutic treatment or clinical trial applications.